Medical device solution expert
Founded in 2003, Envisen specialized in medical oximeters and SpO2 sensors, dedicating our design, research & development, and manufacturing teams in developing products of the highest quality.
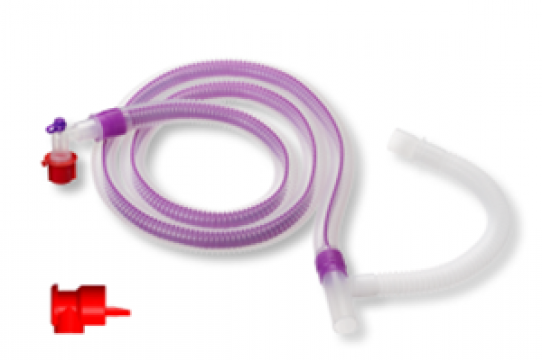
Anaesthesia and Respiratory Circuit Solution
• QTube™ Bilumen Breathing Circuit
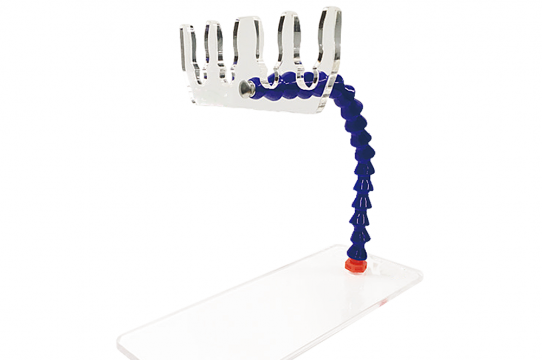
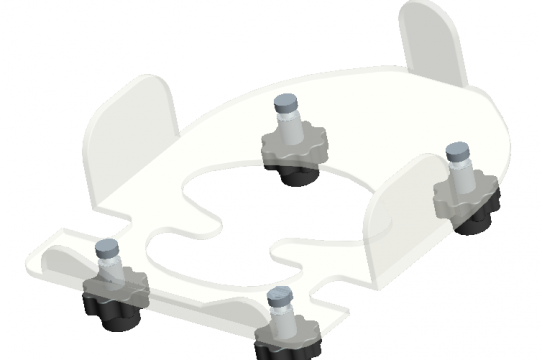
• QView™ Prone Head Positioner
• CoreFlow™ Corrugated Breathing Circuit
• ExtendaFlow™ Extendible Breathing Circuit
• Respiratory Accessories
Patient Monitor Sensor Solution
• SpO2 Sensor and Cables
• Temperature Sensor and Cables
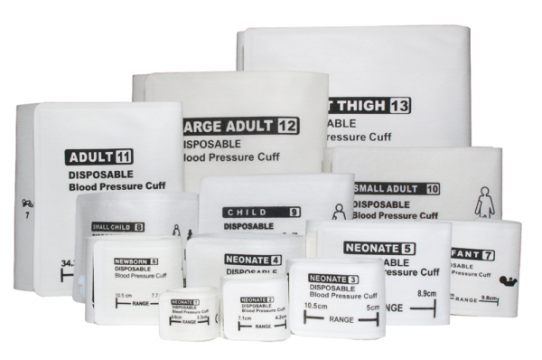
• NIBP Cuffs and Extension Hoses
Pulse Oximetry Monitoring Solution
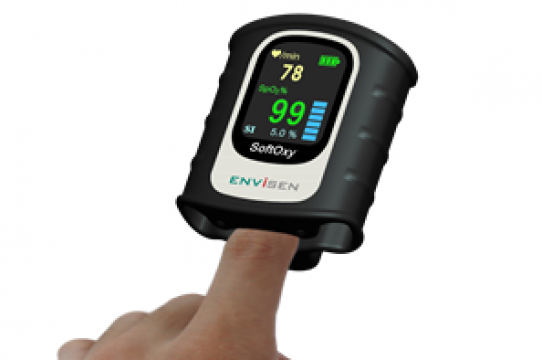
• SoftOxy – Water Resistance Finger SpO2 Monitor
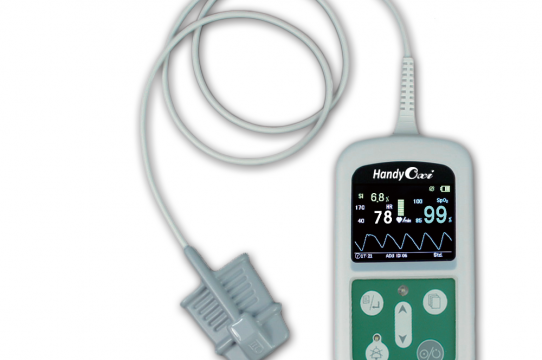
• HandyOxi500 – SpO2 Monitor
• eChip® – The Oximetry module
Alcohol Breathalyzer Solution
• AlcoBoy – The Breathalyzer for Personal Use
• BA1080 – The Breathalyzer For Quick Screening
• BA6060 – The Breathalyzer for Law Enforcement
• Disposable Mouth Piece (S-Type)
Our Services
Envisen’s manufacturing facility is fully audited and accredited for ISO 13485, ISO 9001:2000 and ISO 14000 quality certification by TUV Rheinland Product Safety GmbH.
Download Center
Product Catalogues · · Certificates · Driver Updates